Graphene improving crude oil recovery

Graphene improving crude oil recovery
Lower prices of oil make oil producers struggling and try getting as much oil as possible out of every well has become even more important, but traditional way to drilling and oil recovery grow concerns from nearby residents that some chemicals used to boost production may pollute underground water resources and harms environment.
So researchers from all over the world try to get new way to improve oil recovery, team of researchers gets this goal at the University of Houston, the Texas Center for Superconductivity at UH teamed with researchers from Southwest Petroleum University in Chengdu, China, have come up with a graphene-based method to boost oil recovery, by achieving 15% tertiary oil recovery at a low cost depend on graphene nanomaterials , without the significant volume of chemicals used in most commercial fluids.
The solution – graphene-based Janus amphiphilic nanosheets – is effective at a concentration of just 0.01 percent, meeting or exceeding the performance of both conventional and other nanotechnology-based fluids, said Zhifeng Ren, MD Anderson Chair professor of physics. the best advantage of this way it environmentally friendly and less expensive than options now on the market.
About 75% of recoverable reserves may be left after producers drilling hydrocarbons that naturally rise to the surface or are pumped out mechanically, followed by a secondary recovery process using water or gas injection. Traditional “tertiary” recovery involves injecting a chemical mix into the well and can recover between 10% and 20%, according to many studies. But the large volume of chemicals used in tertiary oil recovery has raised concerns about environmental damage from this chemicals.
In contrast using nanoparticles at low concentration (0.01 wt% or less), show great potential from the environmental and economic perspective.
They describe recovering 15.2% of the oil using their new and simple nanofluid at that concentration – comparable to chemical methods and about three times more efficient than traditional nanofluids.
Graphene Janus nanoparticles either hydrophobic water repelling like oil or hydrophilic
Researchers explain that when graphene-based injected and this fluid meets with the brine/oil mixture in the reservoir, the nanosheets in the fluid spontaneously go to the interface, reducing interfacial tension and helping the oil flow toward surface , the solution helps detach the oil from the rock surface. Under certain hydrodynamic conditions, the graphene-based fluid forms a strong elastic and recoverable film at the oil and water interface, instead of forming an emulsion .
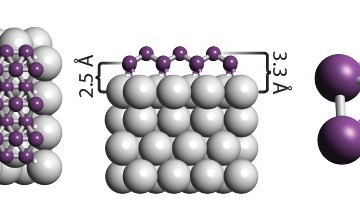
The reason for this difference due to Janus nanoparticles that have at least two physical properties, allowing different chemical reactions on the same particle at 2D material. Nanoparticles are usually either hydrophobic – water repelling, like oil – or hydrophilic, but this material is both Janus and also strictly amphiphilic.
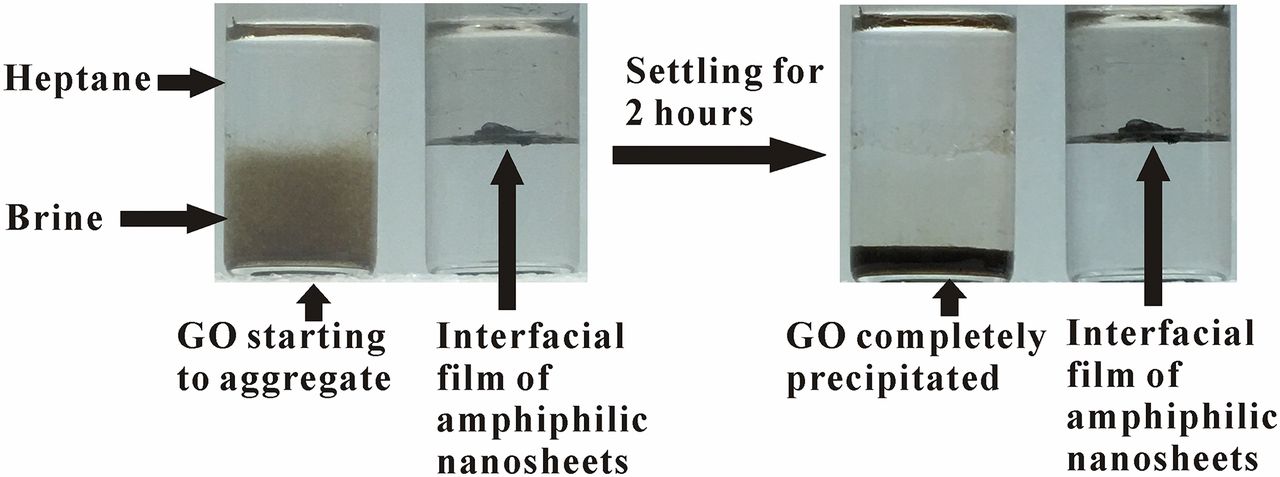
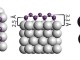
Graphene Oxide (GO) and amphiphilic nanosheets in the heptane/brine system.
Graphene-based Janus particles reactions:
The Janus amphiphilic nanosheets were synthesis produced by tuning the Janus balance of graphene oxide (GO) with alkylamine. Initially, GO was synthesized from chemical oxidation of graphite. Single-surface hydrophobization was then carried out . The nanofluid was made stable to avoid agglomeration of the nanosheets. Brine used in all experiments contained 4 wt % NaCl and 1 wt % CaCl2.
The amphiphilic Janus nanosheets spontaneously accumulated at the heptane/brine interface while GO stayed only in the brine phase and instantaneously started to aggregate. When subjected to vortex-induced vibrations, the amphiphilic Janus nanosheets formed a thin interfacial film separating the heptane and brine in contrast to the GO agglomeration due to the salt screening effect . After settling for 2 h, unmodified GO precipitated while the interfacial film of our nanosheets remained intact. This observation suggested successful asymmetrical functionalization of GO with hydrocarbon chains. which confirmed the successful conjugation of hydrocarbon chains onto the GO surface. Compared with GO, the TGA curve of amphiphilic nanosheets displayed an additional weight loss stage between 400 °C and 500 °C, which may be attributed to the decomposition of the carbon chains